Chapter 2.
Immune Protection of the Process of Fertilization
and Early Preimplantation Embryos.
Early Development of the Secretory Immune System
2.1) Protection of the fertilization process
Fertilization, or sperm-egg fusion, is a critical biological event that occurs in sexually reproducing organisms and is required for tissue organization during their further development. Any interruptions in this process may cause a disturbance in the genome of the newly developed organism, its malformations and even death.
The sperm interacts with three oocyte-associated structures during fertilization: i) the cumulus follicular cell layer surrounding the oocyte, ii) the egg extracellular matrix (the zona pellucida), and iii) the oocyte plasma membrane (1). Each of these interactions is mediated by the sperm head, through proteins both on the sperm surface and within the acrosome, a specialized secretory granule in the form of a small two-layer sack surrounding the head. In gamete fusion, attachment of the two membranes through cell-surface molecules is followed by a physical merging of the plasma-membrane lipids (2). The sperm-egg interaction is accompanied by the activity of different proteins, such as the complement regulator membrane cofactor protein which is synthesized in sperm (3).
Fertilization rate in humans has been shown to be associated with the secretion of seminal Ig antibodies. Sperm-bound IgA antibodies are associated with impaired sperm penetration but exert this impact only when their main location is on the head, while IgM affects fertilization rates when localized simultaneously in both the head and the tail (4,5). In patients with weak to moderate antibody levels, IgA antibodies can significantly reduce a fertilization rate but improve embryo-implantation rate (6). Some cases of man infertility have an immunological basis in sperm proteins: when the state of immune tolerance is disrupted, autoimmunization in the man can cause the appearance of antibodies directed against the sperm antigens with development of hypo-fertility (7). In some cases of autoimmune infertility, these impaired antibodies can result in sperm destruction (8) or premature acrosome loss (9,10).
Increased formation of antibodies against endometrial antigens has been seen in women with infertility caused by endometriosis (11). Such immune changes affect folliculogenesis, ovulation, oocyte quality, early embryonic development and implantation and may be related to alterations within the follicles or oocytes. Expression in unfertilized oocytes of several complement regulators, such as membrane cofactor protein, decay-accelerating factor and protectin, may represent a protective immune mechanism by which the human gametes escape from complement-mediated damage during their travel through the female genital tract (12,13).
2.2) Protection of preimplantation embryos
A series of processes occurs in the oocyte and surrounding membranes during and immediately after fertilization. These processes prevent the penetration of other sperms and create homeostasis for the newly formed organism, the zygote. First, a cortical reaction increases Ca concentration in the zona pellucida, which transforms it into a fertilization membrane, that protects the zygote and preimplantation embryo (conceptus – 14,15) from external (maternal) effects. This type of isolation occurs during the first 3 days after fertilization when the embryo passes through the oviduct. On the 4th day, the embryo enters the uterine cavity at the morula stage with 12 to 16 blastomers and is still surrounded by the zona pellucida. Day 4 is also characterized by the appearance of a cavity inside the embryo and its transformation to the blastocyst. The blastocyst contains a small group of cells (inner cell mass or embryoblast) located at one pole and one layer of flattened cells surrounding the blastocyst cavity and forming its epithelial wall. These cells are called the trophoblast. The zona pellucida is dissolved by uterine secretions and disappears. The embryo is now open to receiving water and nutrition from the uterus and at the same time, it is susceptible to external effects, including those from the mother.
The antigenic status of the preimplantation embryo is ill-defined and there are no clearly recognized maternal immune reactions against this early stage of development. Following implantation, the pregnant female shows evidence of immune recognition of her intrauterine-developing semi-allogeneic conceptus (16). The mammalian maternal immune system reacts to the presence of the conceptus by activating an immune response with its two constituents: a weak rejection reaction and a strong facilitation reaction (17). This immune deviation is modulated by the placenta, its secreted substances and their interaction with decidual cells.
The fate of the human embryo is partly determined by its alloantigenic status. The human preimplantation embryo expresses no MHC antigen, and is thus protected from direct attacks mediated by MHC-restricted T cells. Nevertheless, it may be vulnerable to the adverse effects of some antibodies and cytotoxicity by non-MHC-restricted effector cells (18). Such reactions involve the CD4+ helper T cells and CD8+ MHC-restricted cytolytic effectors and occur at the level of the antigen-presenting cells (APC). Both CD4+ and CD8+ MHC effector T cells can be triggered by the presentation of paternal or embryo-derived peptides by maternal APC. B cells can be triggered by soluble antigens.
There are three threats to the embryo: i) lysis or immobilization by anti-embryonic antibodies, ii) attack by non-MHC-restricted cells, and iii) an ongoing maternal immune response directed towards the implantation site.
In human reproduction, soluble human leukocyte antigen-G (sHLA-G) is considered a possible marker of developmental potential, particularly in embryo implantation (19,20). Its primarily location on the extravillous trophoblast makes this antigen a potential mediator of immune interactions at the maternal-fetal interface during gestation and one of the mechanisms involved in protecting the implanting embryo from rejection by the immunocompetent mother. The human preimplantation embryo expresses several complement regulator proteins associated with the lack of MHC class I antigens that are considered a protective immune mechanism by which the embryo escapes from complement-mediated damage (12,13).
By the 8th day of development, the blastocyst is partially embedded in the endometrial stroma, and its outer layer, the trophoblast, has differentiated into two layers: i) an inner layer of mononucleated cells, the cytotrophoblast, and ii) an outer multinucleated layer, the syncytiotrophoblast, which effects invasion into the uterine wall. The main role in the mother's response to trophoblast invasion in early pregnancy belongs to local immune responses at the maternal-fetal interface (21). It has been hypothesized that specialized mechanisms exist to control access of maternal leukocyte subsets to the decidua and that these mechanisms are modulated during the course of pregnancy. Macrophages and uterine NK cells play different roles in this response (22). CD68+ macrophages, CD56+ lymphocytes and CD3+ T cells are present in the proliferative and secretory endometria. The number of macrophages and CD56+ lymphocytes is dramatically increased at implantation and remains high in the early pregnancy decidua. In contrast to macrophages, CD56+ lymphocytes are more evenly distributed throughout the decidua.
2.3) Components of the secretory immune system in human embryos and early fetuses in the first trimester of pregnancy
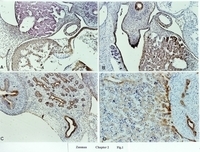
Protein components of the SIS (SC, J chain, and Igs) are present in different organs of the human embryo as early as week 4, and during the whole first trimester of pregnancy (23,24). In 4- to 6-week-old embryos, SC, J chain and IgG are highly reactive in the epithelium of most organs (Fig. 1). In 7- to 8-week-old embryos, SC, J chain, and different Ig subtypes appear in the epithelium of the developing thymus, salivary glands, and metanephric tubules. In 9- to 12-week-old fetuses, the location and rate of reactivity of SC, J chain, and Igs change slightly. In embryos or early fetuses with massive antigenic exposure due to chorioamnionitis, no changes were seen in the distribution or immunoreactivity of SC and J chain, compared to embryos not exposed to massive foreign antigenic attacks (24).
SC immune reactivity decreases or even disappears during development of the pituitary gland and thymus (Fig. 2). A similar picture has been seen in other organs and tissues (24). Matrix cells of the neural tube ependymal layer are SC-positive in 4- to 5-week-old embryos. At weeks 6 to 7, SC is detected in matrix cells in a narrow portion of the neural tube. After week 8 and during the second and third trimesters, SC is found only in the epithelium of the choroid plexuses in the brain ventricles. In the pancreas, 95% to 98% of ductal and acinar epithelial cells originating from the midgut epithelium are SC-immunopositive throughout intrauterine development. In contrast, only occasional Langerhans islet cells, deriving from the acinar epithelium and without acinar free lumen, display weak SC-positive staining up to week 20; thereafter all of them are SC-negative. All of these structures continue to be highly immunopositive for J chain and Igs, even when they become negative for SC (24).
(Color figure)
Fig. 1. A 4-week-old human embryo.*
A. J chain-positive staining in myocardium, endocardium and epicardium (brown staining). The negative staining was seen in the liver. x100.
B. SC-positive staining in the liver (brown staining). SC-negative staining was seen in the myocardium. x200.
C. SC-positive staining in the epithelium of the stomach (s), pancreatic ducts (p), and gall bladder (g), in the coelomic mesothelium. x100.
D. SC-positive staining in hepatocytes (h) and the epithelium of mesonephric tubules (m). x100.
Avidin-biotin complexation and peroxidase technique with commercial markers were used to evaluate various components of the SIS.
* All color figures in this book have been prepared by Prof. P. Gurevich.
.jpg)
Fig. 2.
The number of SC-positive epithelial cells in the pituitary gland (A) and thymus (B)
during human intrauterine development (% to the total number of epithelial cells on a slide) (After ref. 24)




.jpg)

ISO_27001_2013_Hs.jpg)
ISO27799_Hs.jpg)

הרשמה לניוזלטר